Corrective Actions - CAPA Software Systems
Xeeor's Corrective Actions - CAPA Software Systems are designed for Automating the CAPA Process in any Enterprise
Xeeor's CAPA Systems are designed by industry practitioners for automating the Corrective and Preventive Action (CAPA) process in any organization. A CAPA software system is the crux of any quality and compliance process. It is a regulatory requirement that FDA / global regulatory inspectors and ISO auditors consider critical. An automated CAPA system reduces audit time and findings, and decreases risk of product recalls. It improves product quality and safety, increases customer satisfaction, and ensures FDA and ISO compliance.
How can CAPA Software Systems from MasterControl Benefit You?
MasterControl's Corrective Action software is a robust, easy-to-use system designed to effectively manage the corrective action / preventive action process and integrate it with other quality processes critical to regulatory compliance, such as change control, audit, and customer complaints. Here's how the CAPA systems from MasterControl addresses some of the major challenges that companies face in establishing and maintaining effective corrective action and preventive action processes:
Corrective Action Preventive Action CAPA Management Challenges
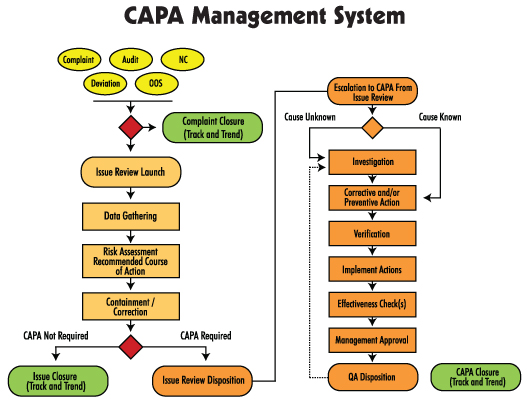
Xeeor CAPA Software System
Inefficient CAPA System for Corrective Action
Paper-based and hybrid systems for Corrective Action and Preventive Action are inexpensive initially. In the long term, however, these systems are inefficient, requiring tremendous man-hours in terms of routing CAPA tasks and other documentation, obtaining approval and signatures, and manual search and retrieval of documents during inspections and audits.
Efficient System for Corrective Action
The Xeeor Corrective Action system automates routing, notification, delivery, escalation and approval of CAPAs and all related documentation. It automates management of entire CAPA process, from initiation to investigation and all the way through closure. Provides a secure, centralized, and Web-based repository for all CAPA documents.
Disconnected CAPA System Processes
A CAPA may be triggered by Form 483 findings, ISO quality audits, customer complaints, or some other source. With manual and hybrid systems, these sources are not connected, making data collection slow and incomplete. Without connectivity, critical information may fall through the cracks, and the root cause investigation is likely to be unreliable.
Connected CAPA Software Systems
The Xeeor Corrective Action software integrates the corrective action and preventive action processes with the rest of the quality system for a holistic approach. For example, the resolution of a corrective action will trigger an engineering change, an SOP change, and retraining of employees on the new SOP.
Poor CAPA Reporting
When customer complaints, deviations, adverse events, and other incidents that can trigger a Corrective Action and Preventive Action are collected manually, there's no guarantee that all critical information will be captured because it is easy to misplace (and time-consuming to update) paper reports. A hybrid system requires re-entering data from hard copy into an electronic system, a process prone to delay and mistakes.
Efficient CAPA Reporting Software System
With the Xeeor CAPA software, a CAPA form can be launched directly from another form (i.e., customer complaint, etc.) to streamline the CAPA process and avoid mistakes during re-entry of data. Links are maintained so users can review a completed process and easily see what triggered the CAPA.
Lack of CAPA Oversight
Poor implementation of CAPA systems a top reason for issuance of a Form 483) may stem from the lack of ability to track and monitor open CAPAs and proactively improve the CAPA process.
Increased Oversight with CAPA Software
The Xeeor Corrective Action software tracks quality incidents that can escalate into a CAPA, such as customer complaints, audit findings, etc. The system provides advanced analytics and reporting capability, including customizable reports and online charting. Through the reports, managers get a real-time view of the CAPA process and can be more proactive about improving their quality system.
CAPA Software Systems Features
Here's a summary of the Xeeor Corrective Action (CAPA) software system and its powerful features:
Best-Practice Forms: Xeeor's Corrective Action System provides best-practice electronic forms and workflow routes that can be used as is or customized based on a company's needs. The CAPA software solution includes:
- An "8D" process to guide the quality team through every step of Corrective Action and Preventive Action (CAPA) implementation, from identification of the problem to investigation of root cause through correction of the problem and prevention of recurrence.
- A Corrective Action and Preventive Action (CAPA) form that can be configured to show the initiator only the relevant information to this step and to require completion only of fields related to data entry.
Connected Quality Processes: Xeeor CAPA software is designed as a "closed loop" solution, streamlining processes, interconnecting different quality subsystems, and tracking quality incidents that can escalate into a CAPA. For example:
- A Corrective Action and Preventive Action (CAPA) form can be launched directly from another form (i.e., customer complaint, audit findings, etc.) to streamline the CAPA process. Links are maintained so users can review a completed process and easily see what triggered the CAPA.
- Relevant data from a form that could potentially require a corrective action is automatically entered into a CAPA form, reducing data entry and eliminating errors from manually transferring information.
- Through the Internet, customers, vendors, and others outside the company can submit a form, such as customer complaint or product issue report, that could lead to a CAPA. Off-site and traveling users can also complete forms pertaining to the Corrective Action and Preventive Action (CAPA) process without being connected to the MasterControl system. They can complete forms offline and then upload.
- Xeeor CAPA software can be integrated with the training application for a more efficient system. A CAPA that causes a change in product design or function will invoke training tasks upon approval of the change. Xeeor CAPA software can automate distribution and grading of online exams, which can be used as proof of personnel competency during FDA inspections or ISO audits
Advanced Analytics and Reporting: With Xeeor, CAPA coordinators can monitor the entire quality management lifecycle, from input to closure. They will get a complete picture of the quality system with the help of the following reporting capabilities:
- Ability to dynamically capture, trend, and link data needed to solve problems, improve processes, and implement preventive measures. A variety of reports (issue summary, aging/overdue, cycle time, etc.) come standard. You can customize reports for issue review, problem prevention, etc.
- Data can be grouped together by a date interval and then charted over a date range. For example, the number of customer complaints can be totaled for each week and charted for the last year. Data can be summarized in multiple levels, so that Corrective Action and Preventive Actions can be reported by product, department, and root cause.
For More Information on CAPA Software Systems
For more information about CAPA software, please feel free to contact a Xeeor representative.
Related Links